Real-world Adherence to Nusinersen in Adults with Spinal Muscular Atrophy in the US: A Multi-site Chart Review Study
Abstract
Limited evidence exists on real-world adherence to nusinersen for the treatment of spinal muscular atrophy (SMA). Data are presented from a multi-site retrospective chart review of 86 adults with SMA initiating nusinersen at nine US centers between January 2017 and February 2019. Seventy-nine (92%) adults remained on nusinersen during the study; 454 (92%) of 493 total nusinersen doses were received on time. Fifty-eight (67%) adults received all nusinersen doses on time. The majority of patients with at least one nonadherent dose resumed nusinersen on time. Most patients followed the dosing schedule across the loading and maintenance dose periods.
INTRODUCTION
Spinal muscular atrophy (SMA) is a rare, severe neuromuscular disorder characterized by progressive muscular atrophy and weakness resulting from motor neuron degeneration [1, 2]. Nusinersen, an antisense oligonucleotide approved for the treatment of SMA across all phenotypes and ages, modulates splicing of survival motor neuron (SMN) 2 pre-messenger RNA to promote production of full-length SMN protein [3]. Nusinersen has shown a favorable benefit:risk profile with significant and clinically meaningful efficacy on motor function and survival endpoints in clinical trials across a broad spectrum of SMA patients [1, 2, 4–7]. Findings from real-world observational studies support the safety and effectiveness of nusinersen in children and adults with SMA [8–14].
Nusinersen is administered intrathecally at 12-mg doses; the approved US regimen begins with 3 loading doses at 14-day intervals and a fourth loading dose 30 days thereafter, followed by maintenance doses every 4 months [2]. Despite its clinical safety and efficacy, there are factors that may potentially affect long-term adherence to nusinersen, including intrathecal administration, provider capacity, payer coverage restrictions, and travel to centers for treatment administrations [14, 15].
Limited evidence exists on real-world adherence to nusinersen including among adult patients with SMA. Retrospective observational cohort studies using US commercial claims databases suggested that real-world adherence to the recommended nusinersen dose schedule was low [16, 17]. However, these studies were based on US commercial insurance claims data, which often have incomplete information on patient medications [18]. To better understand patterns of nusinersen use in US clinics in adults with SMA, a medical chart review study of nusinersen-treated adults was conducted to describe real-world adherence.
MATERIALS AND METHODS
A multi-site retrospective chart review study of nusinersen-treated adults with SMA from 9 US Muscular Dystrophy Association (MDA) clinics was conducted. Individuals with SMA were eligible for inclusion if they were≥18 years of age at time of treatment initiation between January 1, 2017, and February 28, 2019, had a genetically confirmed diagnosis of 5q SMA, and had complete information on the date of nusinersen administration. Patients were followed from the date of initiation until August 31, 2019; discontinuation; or lost to follow-up, whichever was earlier.
Demographic and clinical characteristics were summarized. Discontinuation was defined as having missed 2 consecutive doses based on the expected dosing schedule. Date of discontinuation was considered as the expected date of the first missed dose. The percentage of patients who discontinued during the study period was calculated, and time-to-discontinuation was evaluated using the Kaplan-Meier method [19].
Adherence to the nusinersen dosing regimen was examined using (1) days between doses (i.e., distribution of inter-dose intervals), (2) percentage of patients receiving all doses on time (patient level), and (3) percentage of on time doses while on treatment (dose level) [19]. Expected administration dates were calculated from the actual administration date of the previous dose. Doses were considered not on time using grace periods of±7 days (loading doses) and±28 days (maintenance doses). For patients with≥1 dose not on time, we examined whether they resumed treatment after the initial nonadherent dose.
To understand the potential association between patient characteristics and patterns of nusinersen use, we examined the percentage of patients who discontinued or had≥1 nonadherent dose by baseline characteristics. No statistical testing was conducted due to limited sample size in each subgroup.
RESULTS
Patient characteristics
Nine MDA centers contributed data from 86 adults with SMA. The median (range) age at treatment initiation was 29 (18–68) years (Table 1). Forty-six (53%) of 86 patients had SMA Type III (had attained the maximum motor milestone of independent walking); however, 76 (88%) patients were nonambulatory at baseline, suggesting loss of motor function prior to initiating nusinersen treatment. Fifty-four (63%) of 86 adults with SMA had a record of scoliosis in their medical chart.
Table 1
Baseline characteristics and their association with nusinersen discontinuation and adherence
| All patients (N = 86) | Patients discontinued (n = 7) | Patients with any nonadherent dose (n = 28) | |
| Age at initiation, years, median (range) | 29 (18–68) | 22 (20–57) | 33 (20–65) |
| Female, n (%) | 36 (42) | 2 (29) | 10 (36) |
| SMA type, n (%)a | |||
| Type I | 5 (6) | 1 (14) | 2 (7) |
| Type II | 35 (41) | 4 (57) | 11 (39) |
| Type III | 46 (53) | 2 (29) | 15 (54) |
| Insurance, n (%) | |||
| Commercial | 45 (52) | 4 (57) | 13 (46) |
| Medicaid | 11 (13) | 2 (29) | 2 (7) |
| Medicare | 26 (30) | 1 (14) | 11 (39) |
| Unknown | 4 (5) | 0 (0) | 2 (7) |
| Ambulatory status,b n (%) | |||
| Ambulant | 10 (12) | 0 (0) | 3 (11) |
| Non-ambulant | 76 (88) | 7 (100) | 25 (89) |
| Scoliosis, n (%) | |||
| Yes | 54 (63) | 6 (86) | 17 (61) |
| No | 21 (24) | 1 (14) | 6 (21) |
| Unknown | 11 (13) | 0 (0) | 5 (18) |
| Method of administration (initiation dose), n (%) | |||
| Interspinous lumbar puncture | 60 (70) | 5 (71) | 22 (79) |
| Transforaminal | 8 (9) | 0 (0) | 1 (4) |
| Cervical | 9 (10) | 0 (0) | 3 (11) |
| Other | 8 (9) | 2 (29) | 2 (7) |
| Unknown | 1 (1) | 0 (0) | 0 (0) |
| Invasive respiratory support,c n (%) | |||
| Yes | 14 (16) | 0 (0) | 5 (18) |
| No | 69 (80) | 6 (86) | 23 (82) |
| Unknown | 3 (3) | 1 (14) | 0 (0) |
| Noninvasive respiratory support,d n (%) | |||
| Yes | 23 (27) | 4 (57) | 9 (32) |
| No | 60 (70) | 3 (43) | 19 (68) |
| Unknown | 3 (3) | 0 (0) | 0 (0) |
All percentages are column percentages; percentages may not sum to 100 due to rounding. aSMA Type is a phenotypic classification based on age at symptom onset and maximum motor milestone achieved [23, 24]: in Type I, symptom onset occurs by 6 months of age with no attainment of independent sitting; in Type II, symptom onset occurs from 7–18 months of age and independent sitting is achieved; in Type III, symptom onset occurs at > 18 months of age and independent walking is attained, though individuals may lose this ability over time. bDefined as able to take any steps independently, with or without assistive devices. cTracheostomy and/or ventilator use. dBilevel intermittent positive airway pressure (BiPAP) and/or continuous positive airway pressure (CPAP) use.
Nusinersen discontinuation
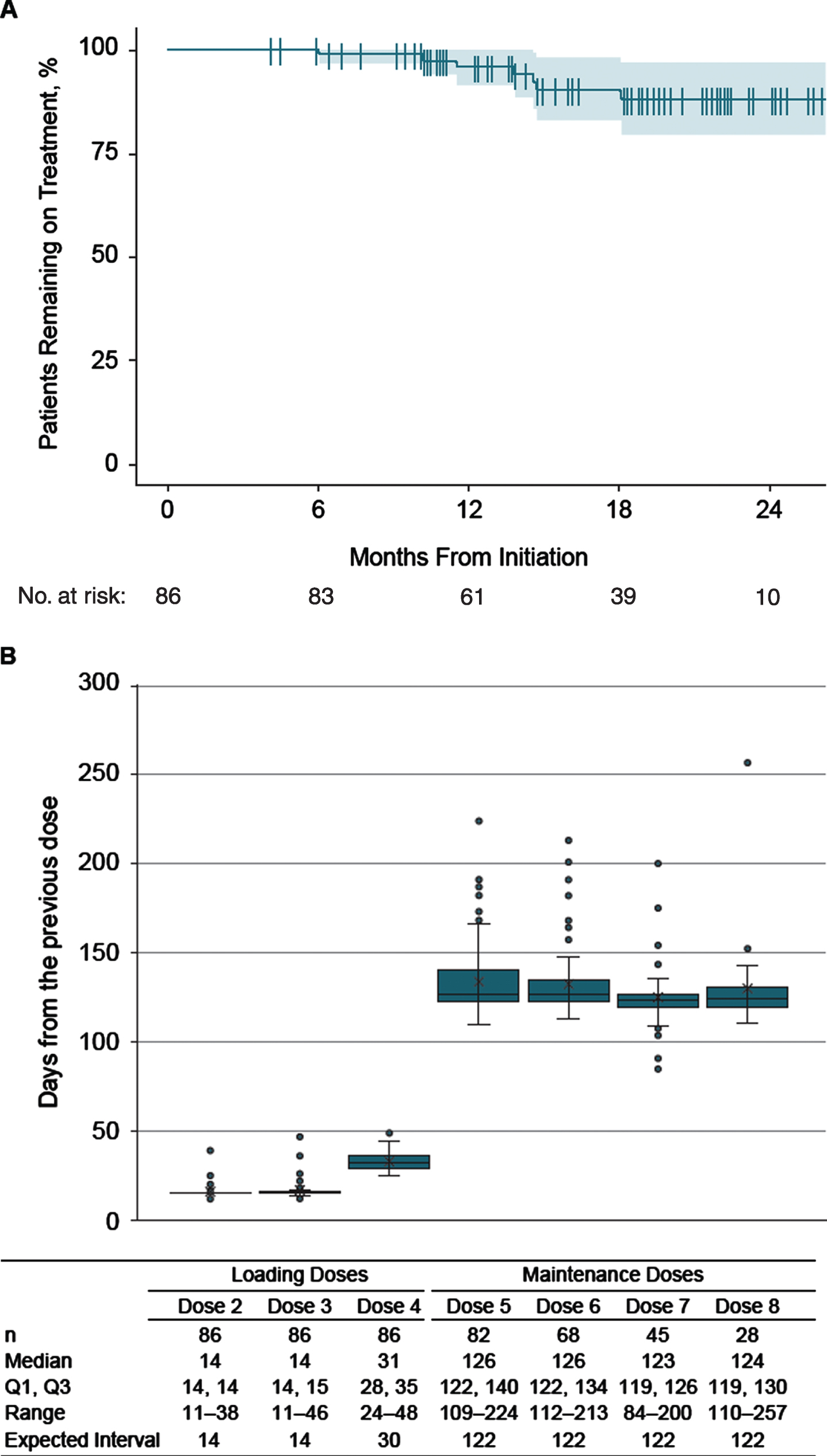
Patients were followed for a median (range) of 1.5 (0.3–2.2) years and had received a median (range) of 7 (4–10) doses. Seventy-nine (92%) adults remained on nusinersen during the study period. All 7 (8%) patients who discontinued nusinersen did so during the maintenance dose phase (Fig. 1A); all of them were nonambulatory and 6 (86%) had scoliosis. None of the patients with invasive respiratory support discontinued (Table 1). None of the 7 patients who discontinued had a record of hospitalizations or emergency room visits while on nusinersen treatment.
Fig. 1
Patterns of nusinersen use over the study period. A: Discontinuation of nusinersen. B: Distribution of inter-dose intervals. Per the US approved regimen, loading doses 2 and 3 are expected to be given at 14-day intervals, with a fourth loading dose 30 days thereafter. Maintenance doses are then administered every 4 months. Note: Data points exceeding a distance of 1.5 times the interquartile range below the 1st quartile or above the 3rd quartile were considered an outlier in the box and whisker plots.
Adherence to nusinersen dosing regimen
The median (range) time intervals between nusinersen dosing during the loading and maintenance periods are shown in Fig. 1B. Most patients followed the dosing schedule across loading and maintenance dose periods. Median time from the previous dose was 14 days for doses 2 and 3, 31 days for dose 4, and 123 to 126 days for doses 5 through 8, which aligned well with the recommended dosing intervals.
A total of 58 (67%) patients received all nusinersen doses on time while on treatment; when examining cumulative doses across all patients, 454 (92%) of 493 doses were received on time (Table 2). Twenty-eight (33%) adults had≥1 dose that was either earlier or later than the expected date. Of these, 26 (93%) continued nusinersen treatment following the nonadherent dose, and 18 (64%) resumed nusinersen on time after the initial nonadherent dose by shifting the subsequent dose by the original interval (i.e., received the subsequent maintenance dose 4 months after the delayed dose). Baseline patient characteristics including ambulatory status, scoliosis, and respiratory support were relatively similar between those with and without≥1 nonadherent dose (Table 1).
Table 2
Summary of follow-up and study outcomes
| Patient-level | n=86 |
| Follow-up time, years, median (range) | 1.5 (0.3–2.2) |
| Number of doses received, median (range) | 7 (4–10) |
| Patients who continuously received treatment, n (%) | 79 (92) |
| Patients who discontinued,a n (%) | 7 (8) |
| Patients receiving all doses on time while on treatment n (%) | 58 (67) |
| Patients with at least one dose that was not on time,b n (%) | 28 (33) |
| Resumed treatment after the initial nonadherent dose,c n (%) | 26 (93) |
| Resumed treatment on time after the initial nonadherent dose,c n (%) | 18 (64) |
| Dose-level | n=493 |
| Doses received on time (dose-level),d n (%) | 454 (92) |
| Doses not received on time (dose-level),d n (%) | 39 (8) |
| Distribution of days deviated (absolute value) for loading doses (n = 14), days,e median (range) | 11 (8–32) |
| Distribution of days deviated (absolute value) for maintenance doses (n = 25), days,e median (range) | 46 (30–135) |
aDiscontinuation defined as having missed 2 consecutive doses based on the expected dosing schedule. bDoses were considered not on time using grace periods of±7 days for loading doses and±28 days for maintenance doses. cCalculated among those with at least 1 dose that was not on time. dCalculated from the second loading dose (i.e., excludes the initiation dose). eCalculated among doses that were not on time.
DISCUSSION
Nusinersen-treated adults with SMA included in this analysis were heterogeneous in age, ranging from 18 to 68 years of age at treatment initiation. Although over half of the adults had SMA Type III, only 12% were ambulatory and the majority had scoliosis, suggesting loss of motor function and disease progression by the time treatment was initiated.
Most patients remained on nusinersen during the study period and received all nusinersen doses on time while on treatment. When examining cumulative doses across all patients, 92% of doses were received on time. Among the adults in this study who experienced any delayed doses, most resumed treatment on time by shifting the subsequent dose by the original interval (e.g., received the subsequent maintenance dose 4 months after a delayed dose). In the event of a delayed or missed dose of nusinersen, a recent pharmacokinetic study demonstrated that administering the delayed dose as soon as possible followed by the subsequent dose on the originally scheduled date was expected to restore targeted exposure levels [20]. Instead of shifting the subsequent dose, patients should return to the original dosing schedule as soon as possible to ensure restoration of optimal exposure to nusinersen.
Recent studies on nusinersen adherence were based on US commercial insurance claims [16, 17], which often fail to capture all medications received by a patient [18]. This may produce erroneous findings when calculating patient adherence [18]. In addition, identifying the time of actual treatment initiation using claims or administrative data has challenges, especially for newly introduced medications. In a recent study of nusinersen adherence based on US claims [17], the authors reported that their study was limited to patients who completed their loading phase (≥ 4 doses). However, their analysis showed that ∼20% of patients were classified as having discontinued after the first month of treatment, which is inconsistent with the loading phase of nusinersen (requiring 2 months to complete). Another adherence study also based on claims data did not use the standard definition of adherence [16]. A recent retrospective study using electronic health records attempted to address these limitations by identifying patients who were followed from their actual initiation date and had similar findings to those reported here with > 90% of doses administered on time [21]. Future real-world studies of nusinersen adherence should carefully assess the potential of incomplete medication information in administrative data.
Our study was based on a medical chart review and included complete information on all doses received by patients at 9 MDA centers. We also separately assessed treatment discontinuation and adherence as recommended by Vrijens et al. [19]. Reasons for discontinuation are unavailable for the small number of patients (n = 7) who stopped nusinersen treatment as this information is not routinely recorded in medical charts. Although the limited subgroup size did not permit the identification of predictors of nusinersen discontinuation or nonadherence using statistical tests, our analysis suggests that the patterns of nusinersen use may not differ substantially by the disease severity. None of the 7 patients who discontinued nusinersen had a record of hospitalizations or emergency room visits while on nusinersen treatment suggesting discontinuation was not due to serious adverse events leading to hospital visits or stays. Our findings on the low rates of discontinuation are consistent with studies conducted during a similar study period to examine nusinersen effectiveness and safety in adult patients treated with nusinersen [8, 11, 22]. The authors of these studies [8, 11, 22] reported that treatment discontinuation occurred due to patient preferences, insurance limitations, lack of perceived effectiveness, and adverse events.
In this study, most patients followed the dosing schedule across the loading and maintenance dose periods. Further studies are needed to better understand short- and longer-term adherence and their relations with patient outcomes. This study contributes toward understanding real-world adherence to nusinersen and could help to evaluate treatment effectiveness in adult patients with SMA receiving care in routine clinical practice.
CONFLICT OF INTEREST
LE: consultant for Biogen and Roche. BY and ADP: employees of and hold stock/stock options in Biogen. CMP: advisory boards and consultant for AveXis, Biogen, and Sarepta; speaker for AveXis and Biogen; Principal Investigator of studies from Astellas, AveXis, Biogen, Catabasis, CSL Behring, Pfizer, PTC, Sarepta, and Scholar Rock. MRF: advisory panel for Biogen; speaker for Allergan and Biogen. SA-D: advisory boards for Amylyx, Biogen, and Orphazyme. MEM: nothing to disclose. DM: nothing to disclose. MSC: Principal Investigator on a study from Scholar Rock. TH-P: consultant or advisor for Biogen, Cytokinetics, Mitsubishi Tanabe Pharma America, Orphazyme, and Samus; site investigator on clinical trials funded by Healey Center, Cytokinetics; Principal Investigator on trials with Mitsubishi Tanabe Pharma America. JMC: nothing to disclose. AC, AK, and MM: employees of Certara, which received consulting fees for the conduct of the study from Biogen.
ACKNOWLEDGMENTS
Biogen provided funding for medical writing support in the development of this report; Yien Liu, PhD, from Excel Scientific Solutions provided writing assistance in the development of the manuscript based on input from authors, and Sarah Becker-Marrero from Alligent Medical Affairs copy edited and styled the manuscript per journal requirements. The authors had full editorial control of the paper and provided their final approval of all content.
REFERENCES
[1] | Darras BT , Chiriboga CA , Iannaccone ST , Swoboda KJ , Montes J , Mignon L , et al. Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies. Neurology (2019) ;92: (21):e2492–e2506. doi: 10.1212/wnl.0000000000007527. |
[2] | De Vivo DC , Bertini E , Swoboda KJ , Hwu WL , Crawford TO , Finkel RS , et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord (2019) ;29: (11):842–856. doi: 10.1016/j.nmd.2019.09.007. |
[3] | Hoy SM . Nusinersen: A review in 5q spinal muscular atrophy. CNS Drugs (2018) ;32: (7):689–696. doi: 10.1007/s40263-018-0545-1. |
[4] | Darras BT , Farrar MA , Mercuri E , Finkel RS , Foster R , Hughes SG , et al. An integrated safety analysis of infants and children with symptomatic spinal muscular atrophy (SMA) treated with nusinersen in seven clinical trials. CNS Drugs (2019) ;33: (9):919–932. doi: 10.1007/s40263-019-00656-w. |
[5] | Finkel RS , Chiriboga CA , Vajsar J , Day JW , Montes J , De Vivo DC , et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: A phase 2, open-label, dose-escalation study. Lancet (2016) ;388: (10063):3017–3026. doi: 10.1016/s0140-6736(16)31408-8. |
[6] | Finkel RS , Mercuri E , Darras BT , Connolly AM , Kuntz NL , Kirschner J , et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med (2017) ;377: (18):1723–1732. doi: 10.1056/NEJMoa1702752. |
[7] | Mercuri E , Darras BT , Chiriboga CA , Day JW , Campbell C , Connolly AM , et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med (2018) ;378: (7):625–635. doi: 10.1056/NEJMoa1710504. |
[8] | Hagenacker T , Wurster CD , Günther R , Schreiber-Katz O , Osmanovic A , Petri S , et al. Nusinersen in adults with 5q spinal muscular atrophy: A non-interventional, multicentre, observational cohort study. Lancet Neurol (2020) ;19: (4):317–325. doi: 10.1016/s1474-4422(20)30037-5. |
[9] | Veerapandiyan A , Eichinger K , Guntrum D , Kwon J , Baker L , Collins E , et al. Nusinersen for older patients with spinal muscular atrophy: A real-world clinical setting experience. Muscle Nerve (2020) ;61: (2):222–226. doi: 10.1002/mus.26769. |
[10] | Walter MC , Wenninger S , Thiele S , Stauber J , Hiebeler M , Greckl E , et al. Safety and treatment effects of nusinersen in longstanding adult 5q-SMA type A prospective observational study. J Neuromuscul Dis (2019) ;6: (4):453–465. doi: 10.3233/jnd-190416. |
[11] | Duong T , Wolford C , McDermott MP , Macpherson CE , Pasternak A , Glanzman AM , et al. Nusinersen treatment in adults with spinal muscular atrophy. Neurol Clin Prac (2021) ;11: (3):e317–e327. doi: 10.1212/cpj.0000000000001033. |
[12] | Maggi L , Bello L , Bonanno S , Govoni A , Caponnetto C , Passamano L , et al. Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J Neurol Neurosurg Psychiatry.- (2020) ;91: (11):1166–1174. doi: 10.1136/jnnp-2020-323822. |
[13] | Coratti G , Pane M , Lucibello S , Pera MC , Pasternak A , Montes J , et al. Age related treatment effect in type II spinal muscular atrophy pediatric patients treated with nusinersen. Neuromuscul Disord. 2021;S0960-8966(21)00075-4. doi: 10.1016/j.nmd.2021.03.012. |
[14] | PeraMC, CorattiG, BovisF, PaneM, PasternakA, MontesJ, et al. Nusinersen in pediatric and adult patients with type III spinal muscular atrophy. Ann Clin Transl Neurol. (2021) ;8: (8):1622–1634. doi:10.1002/acn3.51411. |
[15] | Michelson D , Ciafaloni E , Ashwal S , Lewis E , Narayanaswami P , Oskoui M , et al. Evidence in focus: Nusinersen use in spinal muscular atrophy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology (2018) ;91: (20):923–933. doi: 10.1212/wnl.0000000000006502. |
[16] | Chen E , To TM , Seetasith A , Tan A , Merida M , Iannaccone S . Nusinersen adherence among patients with spinal muscular atrophy in the real world. J Manag Care Spec Pharm (2020) ;26: (S39-S40). |
[17] | Gauthier-LoiselleM, CloutierM, ToroW, PatelA, ShiS, DavidsonM, et al. Nusinersen for spinal muscular atrophy in the United States: Findings from a retrospective claims database analysis. Adv Ther. (2021) ;38: (12):5809–5828. doi:10.1007/s12325-021-01938-w. |
[18] | Cepeda MS , Fife D , Denarie M , Bradford D , Roy S , Yuan Y . Quantification of missing prescriptions in commercial claims databases: Results of a cohort study. Pharmacoepidemiol Drug Saf (2017) ;26: (4):386–392. doi: 10.1002/pds.4165. |
[19] | Vrijens B , De Geest S , Hughes DA , Przemyslaw K , Demonceau J , Ruppar T , et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol (2012) ;73: (5):691–705. doi: 10.1111/j.1365-2125.2012.04167.x. |
[20] | MacCannell D , Berger Z , East L , Mercuri E , Kirschner J , Muntoni F , et al. Population pharmacokinetics-based recommendations for a single delayed or missed dose of nusinersen. Neuromuscul Disord (2021) ;31: (4):310–318. doi: 10.1016/j.nmd.2021.02.014. |
[21] | Johnson N , Wang N , Youn B , Paradis A , Viscidi E , Eaton S , et al. Real-world adherence to nusinersen for the treatment of spinal muscular atrophy (SMA) using US electronic health records. J Manag Care Spec Pharm (2021) ;27: (4-a):S67–S68. |
[22] | Elsheikh B , Severyn S , Zhao S , Kline D , Linsenmayer M , Kelly K , et al. Safety, tolerability, and effect of nusinersen treatment in ambulatory adults with 5q-SMA. Front Neurol. (2021) ;12: :650535. doi: 10.3389/fneur.2021.650535. |
[23] | Finkel R , Bertini E , Muntoni F , Mercuri E , 209th ENMC international workshop: Outcome measures and clinical trial readiness in spinal muscular atrophy 7-9 November 2014, Heemskerk, The Netherlands. Neuromuscul Disord (2015) ;25: (7):593–602. doi: 10.1016/j.nmd.2015.04.009. |
[24] | Lunn MR , Wang CH Spinal muscular atrophy. Lancet (2008) ;371: (9630):2120–33. doi: 10.1016/s0140-6736(08)60921-6. |



